Fda Pfizer Vaccine Booster, Hsftmhv1za Ibm
FDA Vaccine Panel Considers Pfizer-BioNTech Booster Shot Application. FDA Advisory Panel Rejects Widespread Pfizer Vaccine Booster Shots The panel did however endorse the extra shots for those who are 65 or older or at high risk of severe disease.
Advisers to the Food and Drug Administration supported boosters of the Pfizer-BioNTech COVID-19 vaccine for a smaller group of people after they voted against recommending it.
Fda pfizer vaccine booster. Food and Drug Administration on Friday voted against approval of a booster dose of the Pfizer Inc BioNTech SE COVID-19 vaccine. FDA staff declines to take stance on Pfizers Covid booster shots citing lack of verified data Pfizer says Israel data shows third Covid shot restores protection from infection to 95 as it makes. Pfizer received full approval for its vaccine from the FDA so the request to add a booster dose is a supplement to that approval.
September 17 2021 1246 PM. An independent committee advising the FDA voted against Pfizers COVID- 19 vaccine booster shot and vote whether current evidence supports approval for use in people 16 years and older. Members of the regulators vaccine advisory committee voted 16-2 on Friday against endorsing a booster of the two-dose BioNTechPfizer vaccine for Americans aged 16 or older at least six months.
The Food and Drug Administration released a review of Pfizers application for a COVID-19 booster shot on Wednesday saying that although the third shots increased immune responses in study participants the companys vaccine was holding up strongly against severe forms. Pfizer-BioNTech COVID-19 vaccine becomes first to win FDAs full approval paving way for boosters mandates. The Centers for Disease Control and Prevention CDC and the Food and Drug Administration FDA late yesterday contradicted vaccine makers Pfizer-BioNTech on their assertion that people who received the two-dose mRNA vaccine will likely need a third booster dose of vaccine within 6 to 12 months of initial administration.
FDA announced a virtual VRBPAC meeting to discuss the Pfizer-BioNTech supplemental application for a third booster dose of Comirnaty COVID-19 Vaccine mRNA. The Centers for Disease Control and Prevention is planning to convene its own panel of vaccine. FDA approved the first COVID-19 vaccine which has been known as the Pfizer-BioNTech COVID-19 Vaccine and is now marketed as Comirnaty koe.
FILE - A technician inspects filled vials of the Pfizer-BioNTech COVID-19 vaccine. A panel of outside advisers to the US. FDA advisers reject Bidens plan to offer Pfizer boosters for all embraces 3rd shots for older at-risk Americans only.
Pfizer -- and other researchers -- say their studies show people. FDA advisers vote not to recommend Pfizer booster shots for most Americans Vote is a potentially significant blow to the Biden administration after it announced a plan to boost adults. What the FDAs Pfizer booster decision means 0159.
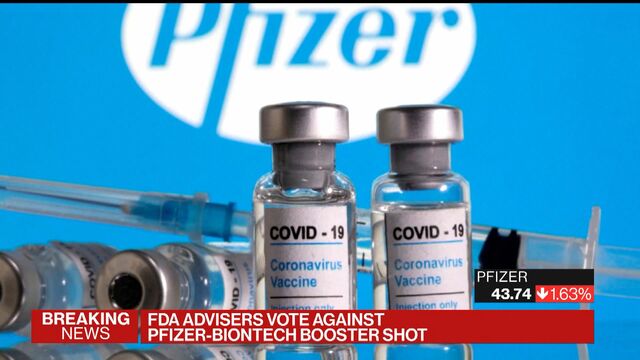
FDA advisory panel rejects widespread Pfizer vaccine booster shots Health Updated on Sep 17 2021 450 PM EDT Published on Sep 17 2021 356 PM EDT. MATTHEW PERRONE and LAURAN NEERGAARD. The votes followed a full day of presentations QA and debate.
Eight months after authorizing the Pfizer-BioNTech COVID-19 vaccine for emergency use.

Fda Scientists Strike Skeptical Tone On Covid Vaccine Boosters At This Time
Fda Advisers Vote To Oppose Pfizer Biontech Covid 19 Vaccine Booster Reuters
Fda Releases Pfizer Covid 19 Booster Shot Data Before Panel Review

Fda Panel Approves Pfizer Covid Vaccine Booster Shots For 65 And Over Nj Com

Fda Strikes Cautious Tone Ahead Of Vaccine Booster Meeting

Fda Panel Approves Pfizer Boosters For People Over 65 At High Risk

Coronavirus Updates Fda Withholds Booster Recommendation Outside Panel To Examine Friday Abc7 New York




Post a Comment
Post a Comment